SafeCoat Medical Inc. protects your medical devices from harmful bacteria and biofilms.
SafeCoat’s technology incorporates self-assembling biocompatible polymers that can be combined with conjugated antimicrobial peptides and applied to virtually any surface as a stable antimicrobial and/or anti-fouling coatings. Of particular interest is the application of this versatile antimicrobial coating to various medical devices and implants that are often the source of biofilm-associated infection. SafeCoat is optimizing methods to manufacture and apply these anti-bacterial coatings to a host of surfaces and can tailor the composition of the coating and associated peptide sequences for any desired application.
Our novel coating features superior:
broad-spectrum antibiofilm and antifouling activity
applicability to a broad range of surfaces and materials to prevent infection, without the need for pre-treatment
excellent stability and longevity once applied to a surface
demonstrated biocompatibility and efficacy in animal models
ability to repel proteins, bacteria and other fouling agents
unique antibiofilm peptides that are incorporated to provide antibiofilm activity in a non-fouling background
Fig 1: Safecoat’s medical device coating technology. The co-polymer tethered peptides kill approaching bacteria and the antifouling surface stops them from adhering and coating the surface of the device. (Image courtesy Dr. Hancock, UBC)
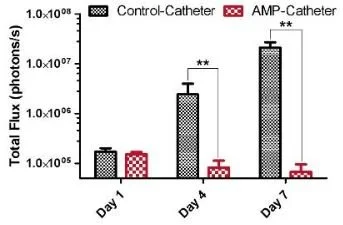
Fig 2. Coated catheter Prevents bacterial growth on a urinary catheter in rats. (Image courtesy Dr. Hancock, UBC)
SafeCoat’s innovative technology is based on a proprietary (patents filed), universal-substrate, independent, covalent coating with demonstrated efficacy against a broad range of pathogens including diverse bacteria (see a recent publication here). The unique structure of the coating (fig. 1) with embedded peptide molecules provides long-term antibiofilm and non-adhesion activity. The coating also repels fouling agents such as proteins and other crystal forming agents. All of the integrated components, except the conjugated antibiofilm peptides, are FDA and Health Canada approved.
The unique combination of a repelling mechanism and non-leaching peptide agent yields effective protection from infection by both repelling bacteria from surfaces and/or killing them without the release of the active antibiofilm peptide agent, resulting in long-term activity.
Pre-clinical testing has demonstrated that the coating is biocompatible and highly stable. Notably, this stability is derived from strong covalent and non-covalent bonds that form with various materials and persist under harsh conditions (e.g. soncation) as well as under either dry or wet storage for several weeks during which the antimicrobial activity was not diminished. The antibiofilm activity of the coating has also been demonstrated in animal models, with a greater than >3-log reduction (>1000-fold reduction) in bacteria recovered from the surface of a catheter using urinary catheter infection model in rats(see Fig. 2)
The coating is low cost, stable, and can be applied in a single dipping or spraying step to a wide range of materials (ex. plastics, glass, ceramics, metals, and cotton). Consequently, it can easily be incorporated into currently available SOPs for the manufacturing of products in relevant applications across multiple settings and industries.
Our patent pending technology was developed by a team of research scientists (Jayachandran Kizhakkedathu, Dirk Lange, Kai Yu and Robert Hancock) at the University of British Columbia.
To learn more about the technology please review our patent application here.